"The amount of energy released when a nucleus undergoes fission can be calculated by determining the net decrease in mass, from the known isotopic masses, and utilizing the Einstein mass-energy relationship. A simple, but instructive although less accurate, alternative procedure is the following. Disregarding the neutrons involved, since they have a negligible effect on the present calculation, the fission reaction may be represented (approximately) by
- Home
- Angry by Choice
- Catalogue of Organisms
- Chinleana
- Doc Madhattan
- Games with Words
- Genomics, Medicine, and Pseudoscience
- History of Geology
- Moss Plants and More
- Pleiotropy
- Plektix
- RRResearch
- Skeptic Wonder
- The Culture of Chemistry
- The Curious Wavefunction
- The Phytophactor
- The View from a Microbiologist
- Variety of Life
Field of Science
-
Simple, atypical but neat estimation of energy released in fission2 weeks ago in The Curious Wavefunction
-
Calcium and Vitamin D Supplements Still Don't Work, New Study Says5 weeks ago in Genomics, Medicine, and Pseudoscience
-
-
-
-
The Site is Dead, Long Live the Site1 year ago in Catalogue of Organisms
-
The Site is Dead, Long Live the Site1 year ago in Variety of Life
-
-
Does mathematics carry human biases?3 years ago in PLEKTIX
-
-
-
-
A New Placodont from the Late Triassic of China5 years ago in Chinleana
-
Posted: July 22, 2018 at 03:03PM5 years ago in Field Notes
-
Bryophyte Herbarium Survey6 years ago in Moss Plants and More
-
Harnessing innate immunity to cure HIV7 years ago in Rule of 6ix
-
WE MOVED!7 years ago in Games with Words
-
-
-
-
post doc job opportunity on ribosome biochemistry!9 years ago in Protein Evolution and Other Musings
-
Growing the kidney: re-blogged from Science Bitez9 years ago in The View from a Microbiologist
-
Blogging Microbes- Communicating Microbiology to Netizens9 years ago in Memoirs of a Defective Brain
-
-
-
The Lure of the Obscure? Guest Post by Frank Stahl11 years ago in Sex, Genes & Evolution
-
-
Lab Rat Moving House12 years ago in Life of a Lab Rat
-
Goodbye FoS, thanks for all the laughs12 years ago in Disease Prone
-
-
Slideshow of NASA's Stardust-NExT Mission Comet Tempel 1 Flyby13 years ago in The Large Picture Blog
-
in The Biology Files
The Curious Wavefunction
Musings on science, history, philosophy and literature
Simple, atypical but neat estimation of energy released in fission
Book Review: "Into Siberia: George Kennan's Epic Journey Through the Brutal, Frozen Heart of Russia", by Gregory Wallance
It may seem hard to believe now, but in 1865, by the time the Civil War ended, Russia was America's best friend in Europe. The two countries enjoyed a healthy diplomatic relationship, buoyed by trade and a mutual distrust of Great Britain; Russia was the only European nation to support the Union during the war. America sent formal condolences when Tsar Alexander was assassinated; Russia did the same when Lincoln was shot.
Jack Dunitz (1923-2021): Chemist And Writer Extraordinaire
Two rare qualities in particular made Dunitz stand out: simple thinking that extended across chemistry, and clarity of prose. He was the master of the semi-quantitative argument. Most scientists, especially in this day and age, are specialists who rarely venture outside their narrow areas of expertise. And it is even rarer to find scientists – in any field – who wrote with the clarity that Dunitz did. When he was later asked in an interview what led to his fondness for exceptionally clear prose, his answer was simple: “I was always interested in literature, and therefore in clear expression.” Which is as good a case for coupling scientific with literary training as I can think of.
Dunitz who was born in Glasgow and got his PhD there in 1947 had both the talent and the good fortune to have been trained by three of the best chemists and crystallographers of the 20th century: Linus Pauling, Dorothy Hodgkin and Leopold Ruzicka, all Nobel Laureates. In my personal opinion Dunitz himself could have easily qualified for a kind of lifetime achievement Nobel himself. While being a generalist, Dunitz’s speciality was the science and art of x-ray crystallography, and few could match his acumen in the application of this tool to structural chemistry.
X-ray crystallography was developed by physicists in the first half of the 20th century to peer inside molecules, the way x-rays and MRI peer inside the human body. Just like those two techniques tell us the locations and structures of various organs in our body, x-ray crystallography tells us where the atoms in a molecule are exactly located, what the lengths of the various bonds are and what the stoichiometry – the exact composition of a complex mixture – is. If you had to point out one technique that has truly revolutionized chemistry, laying the entire chemical universe ranging from rocks and minerals to proteins and nucleic acids bare, it is x-ray crystallography. Dozens of Nobel Prizes for figuring out the structures of increasingly complex molecules, starting with table salt and progressing on through DNA, hemoglobin and the entire ribosome – the multi-component assembly that synthesizes proteins in living organisms – have been awarded through the decades.
One such Nobel Prize was given to James Watson and Francis Crick for figuring out the structure of DNA, a feat made possible by the world-class x-ray crystallography on DNA done by Rosalind Franklin and Raymond Gosling. Dunitz who got his PhD in Glasgow and was working in Oxford in 1953 saw history in the making as he and a colleague drove up to Cambridge to see the ball-and-stick model of DNA using metal plates and tubes that Watson and Crick had constructed. In fact after making a suggestion to Pauling who had figured out the fundamental structure of proteins at Caltech, Dunitz might have contributed an immortal alphabet to the language of life:
While my own work at Caltech had nothing to do with protein structure, Pauling used to talk to me occasionally about his models and what one could learn from them. In his lecture, he had talked about spirals. In conversation a few days later, I told him that for me the word “spiral” referred to a curve in a plane. As his polypeptide coils were three-dimensional figures, I suggested they were better described as “helices.” Pauling’s erudition did not stop at the natural sciences. He answered, quite correctly, that the words “spiral” and “helix” are practically synonymous and can be used almost interchangeably, but he thanked me for my suggestion because he preferred “helix” and declared that he would always use it henceforth. Perhaps he felt that by calling his structure a helix there would be less risk of confusion with the various other models that had been proposed earlier. In their 1950 short preliminary communication, Pauling and Corey wrote exclusively about spirals, but in the series of papers published the following year the spiral had already given way to the helix. There was no going back. A few years later we had the DNA double helix, not the DNA double spiral.
After seeing the power of crystallography to crack open the very structure of life, Dunitz spent the rest of his career in that field at the famed ETH in Zurich, capping an incredible 64-year-long career with his death in 2021; his last paper, written when he was 96, was appropriately a critique of certain chemical terminology and titled “Bad Language“.
Dunitz was truly unusual in ranging across the broad spectrum of chemical disciplines. Organic, inorganic and biological chemistry all came within his purview, aided by the powerful interdisciplinary generality of the tool of x-ray crystallography which he wielded with aplomb. Over his long career he published more than 350 scientific papers and penned several foundational books. It would be impossible to review his entire corpus, so I now review three of his papers which made a striking impression on me, which I have cited and read many times over the years, and which I think showcase his striking originality in marshaling simple models and arguments across a variety of fields.
Perhaps my favorite paper of Dunitz’s is a 1997 paper titled “Organic Fluorine Hardly Ever Accepts Hydrogen Bonds”. Some explication is needed here. Hydrogen bonds are weak, fleeting bonds between hydrogen and other atoms which, while weak, are absolutely critical in keeping all kinds of molecules including proteins and nucleic acids together. In fact, water would not be a liquid without hydrogen bonds and life as we know it would not exist without them. It is their very transient nature that make hydrogen bonds “on-demand” bonds; they can be formed when needed and rapidly dissolved when no longer needed. Linus Pauling, often considered the most important chemist of the 20th century, had underscored the importance of hydrogen bonds in the 1930s in his seminal book, “The Nature of the Chemical Bond”. Typically hydrogen bonds are formed between hydrogen and what are called ‘electronegative’ atoms, ones like oxygen and nitrogen. Electronegative atoms have a particular affinity for electrons, attracting the electron clouds of atoms like hydrogen; the most common hydrogen bonds therefore are ones between oxygen and nitrogen.
There is another element on the periodic table, a most unusual one, which should be even more powerful at forming hydrogen bonds, except that it isn’t. That element is fluorine. Fluorine is in fact the most electronegative element on the periodic table, which is why we would expect it to form hydrogen bonds with furious abandon. But while inorganic fluorine found in compounds like hydrofluoric acid – a diabolically corrosive and dangerous substance – does form these hydrogen bonds, organic fluorine (fluorine bonded to carbon, that is) found in compounds like polytetrafluoroethylene – PTFE or Teflon – does not. In fact it is precisely fluorine’s reluctance to form hydrogen bonds with water in Teflon that makes it such an effective coating for non-stick cookware.
This behavior of fluorine is what the facts indicate, but the facts in this case don’t line up well with chemical theory which expects hydrogen bonding tendencies to increase with electronegativity. Fortunately there is a big database of “solved” crystal structures of organic molecules that includes molecules containing fluorine; it was only waiting for the right person to come along to interpret it. Dunitz’s paper was perhaps the first one to exhaustively analyze this database and then come up with a convincing chemical explanation for the counterintuitive observation that fluorine hardly ever forms hydrogen bonds. He looked at almost 6000 structures with fluorine and determined that hardly a dozen form hydrogen bonds between the fluorine and other hydrogen atoms. The details of why fluorine is reluctant to form hydrogen bonds is beyond the scope of this post (and explained in a further paper by Dunitz), but the qualitative explanation is simple: imagine that an electronegative element like oxygen has “hands” that pull others toward it. The problem with fluorine is that it is so electronegative that it simply keeps its hands to itself.
Even today I keep meeting chemists who, based on what seems like entirely sound chemical logic, expect fluorine to form hydrogen bonds. They recommend that one make drug molecules with fluorine that would enable them to stick better to and form hydrogen bonds with proteins that they want to block, proteins that have gone haywire in cancer, for instance. It is then that I find myself waving Dunitz’s paper – sometimes literally since I still “believe” in paper copies – with the fervent enthusiasm of a preacher.
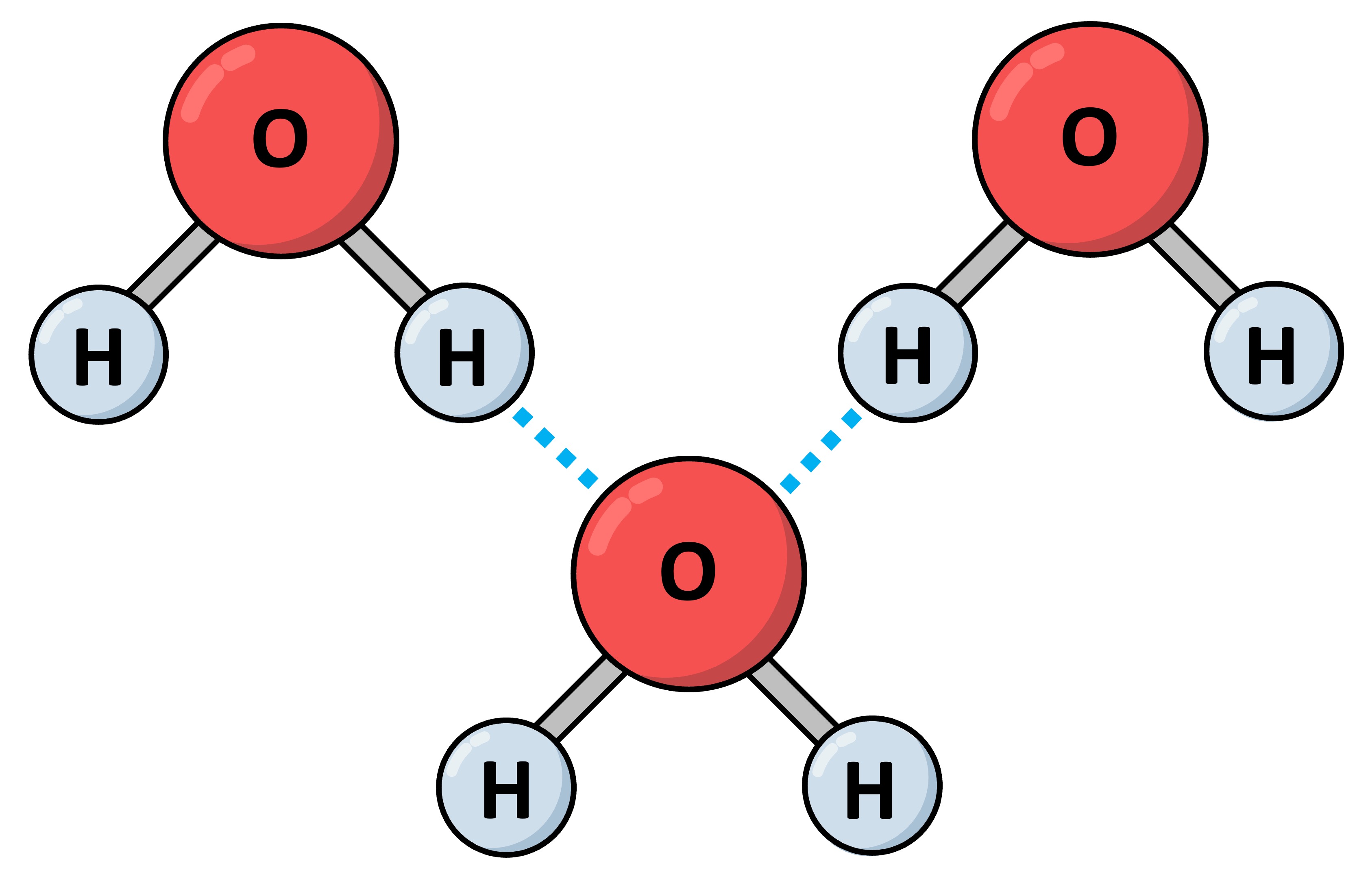
The second paper from Dunitz that I often highlight shows Dunitz’s masterful application of simple, semi-quantitative arguments to addressing an important question. One of the most important things that scientists want to know when thinking about biological molecules like proteins is how they interact with water. All biological molecules are swimming in a vast sea of water; in fact water not just ubiquitously surrounds these molecules but is also an intimate participant in their behavior. Knowing the thermodynamics of this system – the strength of binding in particular between proteins and other molecules and water – is critical in engineering better drugs and proteins. Two factors are key in quantifying this binding: enthalpy and entropy. Roughly speaking, enthalpy concerns itself with the strength of the interactions between two molecules and entropy concerns itself with how loosely or tightly they bind, whether they stay in place or whether they jiggle around. While enthalpy is often easy to estimate, entropy is not.

In 1994, Dunitz wrote a one-page paper in the journal ‘Science’ titled “The Entropic Cost of Bound Water Molecules in Crystals and Biomolecules” in which, using the simplest of data and arguments, he came up with a reliable number quantifying the entropy of a single water molecule binding to biological molecules. One of his strengths here which is also showcased in the fluorine paper is his ability to look at old data and come up with new explanations. He starts by looking at data on hydrates, simple salts like zinc sulfate which are surrounded by water molecules. He also looks at old data on the thermodynamics of the melting and freezing of ice which would also gives estimates on the entropy of water molecules; he points out something telling which is now a far more serious problem in our specialized world, namely that “this information has been available for a long time, but science has become so specialized that its practitioners in one branch are all too often unaware of what is common knowledge in another.”
How is thermodynamic information on ice, liquid water and hydrate salts relevant to what goes on with proteins? Because, as Dunitz astutely observes, this thermodynamics sets an upper limit on the entropy question for water around proteins: salts bind water molecules most tightly, so surely proteins would bind them more weakly? Using these arguments, Dunitz arrives at a value for the entropy of a bound water molecule which is now commonly used in calculations. The paper demonstrates characteristic Dunitzian strengths which should be widely emulated: scrupulous attention to existing data, including data going back decades, simple back-of-the-envelope calculations, and proof by analogy.
The last paper among Dunitz’s great corpus of works is a paper which exemplifies a particularly fine example of speculative as well as interdisciplinary thinking. It questioned a fact which everyone knows but no one really thinks about: Why is body temperature for animals like humans who can maintain their temperature about 36 degrees celsius, and why is it maintained across such a huge range of organisms? As we know, unless they are sick, homeothermic animals like ourselves are very efficient at regulating body heat. An explanation provided by some previous scientists pointed to the specific heat of water. Specific heat is the amount of heat required to change the temperature of a substance by one degree. Water has a very large specific heat compared to many other substances, which is just one of many of its remarkably unusual properties. But this specific heat happens to reach its lowest value at about 36 degrees celsius, just the optimum temperature mentioned above. The previous explanation said that water at this temperature was least resistant to changes in its temperature and quickly dissipated whatever heat was added to or subtracted from it.
Dunitz and his co-author, Steven Benner, found this argument “appealing, but not correct” in their response, published in the journal Nature in 1986. First, they identify what seems to be an obvious but overlooked problem: the smaller the specific heat, the easier it will be to cause fluctuations in temperature, making it harder for an organism to survive, not easier. They also realize that the previous argument only applies to pure water; water in living organisms is a complex aqueous mixture consisting of water, biomolecules like proteins and salts. So what could be responsible for the precise temperature regulation? Dunitz and Benner don’t pretend to know the answer, but they focus on two of water’s unique properties in particular, its hydrophobicity (or tendency to repel greasy, oil-like substances) and its viscosity. As temperature rises, water becomes less viscous and therefore facilitates chemical reactions in it. However, hydrophobicity also lessens with temperature, which could lead to unwanted mingling between water and greasy substances. Dunitz and Benner speculate that a temperature of 36 degrees is a Goldilocks-like zone, one where the viscosity is low enough for chemical reactions to speedily occur but hydrophobicity is high enough to prevent greasy substances from dissolving too easily.
To me this paper is a superb example of informed speculation, not pretending to solve a problem but offering a tantalizing potential solution and gently but firmly demolishing an existing explanation. It is widely believed that life anywhere in the universe would have to be based on water. Dunitz and Brenner’s analysis of the temperature dependence of water’s unique viscosity and hydrophobicity provides another window on why this substance is so unique for supporting life.
These three papers may serve to exemplify the range of Dunitz’s contributions, and they are but a slice of his vast corpus. In another analysis, he used a purely mathematical argument about the geometry of a pentagon to predict the experimentally-verified geometry of cyclopentane, a molecule with five carbon atoms arranged in a ring. His is a textbook name in many ways, none more so than in the eponymous “Bürgi-Dunitz angle” which describes the angle of attack of a reacting molecule and the precise geometric configuration of the reactants in an important class of organic reactions, one which has yielded great dividends of both academic and industrial interest.
 Apart from scientific papers spanning a remarkable variety of topics, Dunitz also wrote books that are considered foundational in the field. Perhaps my favorite book of his is written for laymen. “Reflections on Symmetry: In Chemistry…and Elsewhere“, written with his co-author Edgar Heilbronner, is a marvelous look at symmetry, perhaps the deepest quality of nature. Symmetry is absolutely fundamental not just for chemistry and biology but in the deepest reaches of physics, including quantum mechanics and particle physics. Dunitz and Heilbronner’s book is a romp through aspects of symmetry in fields as disparate as medieval mathematics, Islamic and modern art and of course, chemistry. It is a beautiful book, filled with illustrations and elegant arguments.
Apart from scientific papers spanning a remarkable variety of topics, Dunitz also wrote books that are considered foundational in the field. Perhaps my favorite book of his is written for laymen. “Reflections on Symmetry: In Chemistry…and Elsewhere“, written with his co-author Edgar Heilbronner, is a marvelous look at symmetry, perhaps the deepest quality of nature. Symmetry is absolutely fundamental not just for chemistry and biology but in the deepest reaches of physics, including quantum mechanics and particle physics. Dunitz and Heilbronner’s book is a romp through aspects of symmetry in fields as disparate as medieval mathematics, Islamic and modern art and of course, chemistry. It is a beautiful book, filled with illustrations and elegant arguments.
Jack Dunitz was one of those scientists who enrich everything they touch, across a wide range of domains, with insight, revelation and beauty. The simplicity and importance of his arguments, humility as a man and fearlessness in tackling disparate problems will be a candle that will keep lighting the minds of aspiring chemists and other scientists for eons to come.
How Niels Bohr predicted Rydberg atoms
In Niels Bohr's original 1913 formulation of the quantum atom, the Bohr radius r was proportional to n^2, n being the principal quantum number. Highly excited states would correspond to very large values of n and Bohr predicted these "giant" atoms would exist. Since the volume scales as r^3 or n^6, for n=33 you should see a "hydrogenic" atom a billion times larger than a ground state hydrogen atom. However, no spectral lines corresponding to such atoms were observed. So was Bohr's theory wrong?

Galton's "Hereditary Genius" (1871)
As someone who loved collecting vintage books, I was stoked to acquire a first American edition of Francis Galton's pioneering book “Hereditary Genius” for the bizarrely low price of $25 - most copies in good condition like this one sell for an unaffordable few hundred dollars at the minimum.
John Polkinghorne's "Belief in God in an Age of Science"
A book I have been enjoying recently is John Polkinghorne's "Belief in God in an Age of Science." Polkinghorne who died recently was a noted theoretical physicist who was also a theologian. Unlike Polkinghorne I am an atheist, but he makes a good case for why religion, science, poetry, art, literature should all be welcomed as sources for truth about the universe and about human beings. A quote I particularly like from it:
Tolman, “The Principles of Statistical Mechanics, Chapter 1, Part 1
Survey of classical mechanics: Generalized coordinates and momenta. Lagrangian equations. Derivation of Hamilton’s equations from Lagrangian. Poisson brackets. Hamilton as representing invariant E under time for conservative systems.
“Pull quote”: Something simple and seemingly obvious but actually deep and foundational
Some notes (not checked for typos!)
100 Desert Island Books
Finally got around to making that "100 books I would want on a desert island" list. Another title would be "100 books that I consider essential reading for *my* life": thus, this is a personal selection. I don't claim to have this list cover the most important aspects of human life or the universe, nor do I expect "famous" books to be on this list (although some of them are). The list just reflects my personal traditional interests - history and philosophy of science has the most numbers, followed by science textbooks, general history, philosophy and theology and a tiny sliver of fiction (I started reading fiction seriously quite recently). One condition in listing these books was that I should have read them in their entirety: this is true of all of them except "Gödel, Escher, Bach" which I think I am going to keep soldiering through my whole life. I am very privileged to call some of the authors here my friends.
One common thread running through most of these books is that I discovered them early, when I was in high school, college and graduate school, in most cases in either the college or university library or the British Library which was a stone's throw from where I grew up. Early impressions are often the strongest, so I keep coming back to these volumes and they keep inspiring and instructing me.
I have thousands of books on my shelf and I always find it hard to give any away. There are many others I haven't listed here which I love, but if I actually had just these 100 (110 to be precise), I wouldn't be entirely depressed (just don't tell my significant other...).
HISTORY AND PHILOSOPHY OF SCIENCE (INCLUDING BIOGRAPHY AND AUTOBIOGRAPHY)
Richard Rhodes - The Making of the Atomic Bomb
Richard Rhodes - Dark Sun: The Making of the Hydrogen Bomb
Freeman Dyson - Disturbing the Universe
Freeman Dyson - Infinite in All Directions
George Dyson - Turing’s Cathedral
George Dyson - Darwin Among the Machines
Edward Wilson - Naturalist
Edward Wilson - Consilience
James Gleick - Chaos
John Horgan - The End of Science
Robert Serber - Peace and War
Jeremy Bernstein - Hans Bethe: Prophet of Energy
Silvan Schweber - In the Shadow of the Bomb
Silvan Schweber - QED and the Men Who Made It
David Kaiser - Drawing Theories Apart
Kip Thorne - Black Holes and Time Warps
Robert Kanigel - The Man Who Knew Infinity
Robert Hoffman - The Man Who Loved Only Numbers
Robert Crease and Charles Mann - The Second Creation
Douglas Hofstadter - Gödel, Escher, Bach
Alice Kimball-Smith and Charles Weiner - Robert Oppenheimer: Letters and Recollections
Peter Galison - Image and Logic
Emanuel Derman - My Life as a Quant
Kameshwar Wali - Chandra
John Gribbin - In Search of Schrödinger’s Cat
John Casti - Paradigms Lost
John Casti - The Cambridge Quintet
John Casti - Gödel: A Life in Logic
George Johnson - Strange Beauty
Roger Penrose - The Emperor’s New Mind
Roger Penrose - The Road to Reality
Richard Dawkins - Climbing Mount Improbable
Gerald Durrell - My Family and Other Animals
Konrad Lorenz - King Solomon’s Ring
Robert Laughlin - A Different Universe
Horace Freeland Judson - The Eighth Day of Creation
Peter Michelmore - The Swift Years: The Robert Oppenheimer Story
Richard Feynman - Surely You’re Joking Mr. Feynman
Stanislaw Ulam - Adventures of a Mathematician
Laura Fermi - Atoms in the Family
Werner Heisenberg - Physics and Philosophy
Ronald Clark - Einstein
Steven Pinker - The Blank Slate
David Deutsch - The Beginning of Infinity
Steven Weinberg - Dreams of a Final Theory
J. Robert Oppenheimer - The Open Mind
Stuart Kauffman - Reinventing the Sacred
Barry Werth - The Billion Dollar Molecule
Oliver Sacks - On the Move
Carl Sagan - The Demon-Haunted World
Max Perutz - I Wish I’d Made You Angry Earlier
Jonathan Allday - Quarks, Leptons and the Big Bang
Philip Ball - H2O: A Biography of Water
Philip Ball - The Self-Made Tapestry
Alan Lightman - Einstein’s Dreams
Alan Lightman - The Accidental Universe
Brown, Pais and Pippard - Twentieth Century Physics (3 volumes)
Ed Regis - Who Got Einstein’s Office?
C. P. Snow - The Physicists
TEXTBOOKS
Ira Levine - Quantum Chemistry
Peter Atkins - Molecular Quantum Mechanics
Lubert Stryer - Biochemistry
Albert Lehninger - Biochemistry
George Simmons - Introduction to Topology and Modern Analysis
George Simmons - Differential Equations
Richard Feynman - The Feynman Lectures on Physics
David Griffiths - Introduction to Electrodynamics
John Lee - Inorganic Chemistry
Samuel Glasstone - Sourcebook on Atomic Energy
Samuel Glasstone - Thermodynamics for Chemists
Arthur Beiser - Concepts of Modern Physics
Gautam Desiraju - The Weak Hydrogen Bond
Linus Pauling - The Nature of the Chemical Bond
Linus Pauling and Edward Bright Wilson - Introduction to Quantum Mechanics
Clayden, Warren, Reeves and Wothers - Organic Chemistry
Eric Anslyn and Dennis Dougherty - Modern Physical Organic Chemistry
Wells, Wells and Huxley - The Science of Life
Goodman and Gilman - The Pharmacological Basis of Therapeutics
Jerry March - Advanced Organic Chemistry
HISTORY
Barbara Tuchman - The Guns of August
William Shirer - The Rise and Fall of the Third Reich
James Swanson - Manhunt: The 12-Day Chase for Lincoln’s Killer
David McCullough - Truman
James Scott - Against the Grain
James McPherson - Battle Cry of Freedom
Gordon Wood - Empire of Liberty
John Barry - Roger Williams and the Creation of the American Soul
Bernard Bailyn - The Ideological Origins of the American Revolution
Robert Caro - The Years of Lyndon Johnson (Vols. 1-4)
Rick Atkinson - An Army at Dawn
Will Durant - Our Oriental Heritage
Russell Shorto - The Island at the Center of the World
Nick Bunker - An Empire on the Edge
Brad Gregory - Rebel in the Ranks
Cornelius Ryan - The Longest Day
PHILOSOPHY AND THEOLOGY
Sam Harris - The End of Faith
David Edmonds and John Eidinow - Wittgenstein's Poker
Plato - The Republic
Matthew Stewart - The Courtier and the Heretic
Isaiah Berlin - The Proper Study of Mankind
Bertrand Russell - Unpopular Essays
Bertrand Russell - Why I am Not a Christian
FICTION
Vasily Grossman - Life and Fate
Haruki Murakami - What I Talk About When I Talk About Running
Cormac McCarthy - Blood Meridian
Cormac McCarthy - The Road
Isaac Asimov - Asimov’s Mysteries
Cordwainer Smith - No, No, Not Rogov! (this is a single story but it is very striking in its vividness and poetry and made a deep impression)
Leo Tolstoy - War and Peace
Fyodor Dostoevsky - Notes from the Underground
William Faulkner - As I Lay Dying
H. G. Wells - The Time Machine
Chekhov - Stories
Brenner, von Neumann and Schrödinger
Erwin Schrödinger's book, "What is Life"?, inspired many scientists like Crick, Watson and Perutz to go into molecular biology. While many of the details in the book were wrong, the book's central message that the time was ripe for a concerted attack on the structure of the genes based on physical principles strongly resonated.
However, influence and importance are two things, and unfortunately the two aren't always correlated. As Sydney Brenner recounts in detail here, the founding script for molecular biology should really have been John von Neumann's 1948 talk at Caltech as part of the Hixon Symposium, titled "The General and Logical Theory of Automata". In retrospect this talk was seminal and far-reaching. Brenner is one of the very few scientists who seems to have appreciated that von Neumann's influence on biology was greater than Schrödinger's and that von Neumann was right and Schrödinger wrong. Part of the reason was that while many biologists like Crick and Watson had read Schrödinger's "What is Life?", almost nobody had read von Neumann's "General and Logical Theory of Automata".






























